SI base unit: mole (mol)
The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly
The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
This definition implies the exact relation NA =
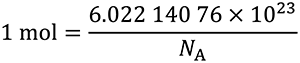
The effect of this definition is that the mole is the amount of substance of a system that contains
See:
SI Brochure - Appendix 2
SI Brochure - excerpts from Appendix 4