SI base unit: kelvin (K)
The kelvin, symbol K, is the SI unit of thermodynamic temperature. It is defined by taking the fixed numerical value of the Boltzmann constant k to be
This definition implies the exact relation k =
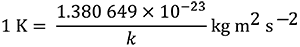
which is equal to
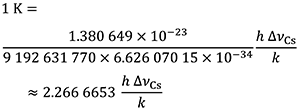
The effect of this definition is that one kelvin is equal to the change of thermodynamic temperature that results in a change of thermal energy k T by
See:
SI Brochure - Appendix 2
SI Brochure - excerpts from Appendix 4