Knowledge Transfer Study: Purity Evaluation of Tetracycline.HCl
Goal
To transfer knowledge and practical experience on characterization methods for pure veterinary drug materials for the development of primary reference materials and calibration solution standards for drug residue measurements in food.
Participation
NMIs and DIs from Member States and Associates wishing to participate in the knowledge transfer study should complete the registration form to receive further details.
Introduction
As part of its Metrology for Safe Food and Feed CBKT programme related to Pesticides and Veterinary Drug Residues, the BIPM, in collaboration with UME, is launching a knowledge transfer study on Tetracycline.HCl purity evaluation. The activity is organized in two parts, the first being the development of a purity evaluation guideline (PEG), the second will be training using the guideline and associated material.
Participation in Stage 1 of the knowledge transfer study, starting in 2021, is open to:
| 1A. | NMIs with established capabilities in pure organic material characterization as demonstrated by CCQM Organic Analysis Working Group (OAWG) key comparison participation or who have Calibration and Measurement Capabilities (CMCs) in this area and who are willing to contribute measurements and method descriptions to the development of a PEG on tetracycline hydrochloride (TET.HCl). The combined characterization data provided by the participants will be collated and reviewed and used to prepare a PEG which will be made available as an open-access document on the BIPM website. |
| 1B. | NMIs or DIs actively developing capabilities in Organic Pure Material characterization, who want to participate on a “learn-as-you-measure” basis for capacity development purposes and wish to be able to review their results against laboratories with established capabilities in the field. |
Stage 2 of the activity will be run as an on-line training course based on the developed PEG and tetracycline hydrochloride (TET.HCl) material, starting in 2023.
Laboratories developing qNMR methods in either STAGE 1 or 2 of the study will also be provided with four internal standard reference materials, INMETRO CRMs donated to the BIPM for use in the CBKT programme, (Maleic Acid, Dimethylsulfone, Potassium Hydrogen Phthalate (KHP) and Dimethyl Terephthalate). Reference documents and guidelines on the use of the qNMR standards are published and available here.
The knowledge transfer study is a capability building and knowledge transfer activity. Laboratories wishing to demonstrate competencies in organic purity characterization are able to do this only through participation in CCQM and Regional Key comparisons.
Measurand
Participants in the study will be provide with individual units, containing 500 mg of solid powder, consisting of high purity tetracycline hydrochloride salt.
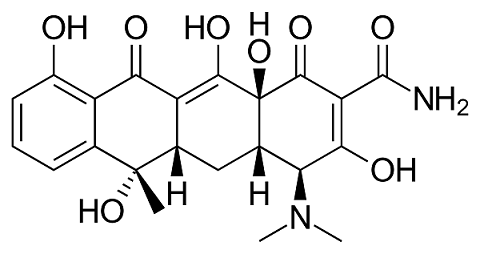
.HCl
The material is known to be stable as supplied. Initial studies have shown that it is also stable and of sufficient homogeneity when taken into solution provided suitable conditions are used and appropriate precautions are followed when handling solutions containing the material. Information on best practice for handling solutions will be provided to participants in the activity.
Measurements
STAGE 1A: Participants contributing to PEG development
This level of participation is recommended for laboratories that have participated in CCQM OAWG purity key comparisons or have recognised CMCs in this area.
Participants are required to analyze and provide the BIPM with results and written descriptions of each method they will apply for TET.HCl material characterization. Measurements and methods used should contribute to the ability to determine the mass fraction of tetracycline present as the free base in the material, either by an indirect, “mass balance” approach involving summation of the impurity components present or by a direct qNMR assignment of the tetracycline content. Information is also requested to provide results and methods that can be used to assign the mass fraction of tetracycline present as the hydrochloride salt in the material.
Participants are not required to undertake a full assignment of the tetracycline content of the material. However, the combination of all results and methods submitted should allow the BIPM to produce a comprehensive PEG for the material. The measurements undertaken by individual participants should include some of the following:
- tetracycline free base content by qNMR
- total related structure impurity content by LC-based methods
- water content by Karl Fischer titration
- water content by other methods
- chloride and related anionic impurity content
- volatile organic content (VOC)
- total non-volatiles impurity content by TGA or ashing techniques
- metal ion impurity content by IC, ICP-MS or other techniques
- hygroscopicity (adsorption of water as a function of relative humidity)
Participants are free to report the results of other measurements they consider relevant to the overall goal of the study, including measurements (NMR, MS, UV) that can be used to establish the qualitative structure of the material.
In each case measurement results must be accompanied by a clear method description at a level of detail that could be incorporated into a Guideline.
Further information
Further information on the study can be obtained by contacting the BIPM.
STAGE 1B: Participation on a “learn-as-you-measure” basis
This level of participation is intended for laboratories that are actively considering or are developing purity assignment capabilities but have not or not regularly participated in CCQM OAWG purity comparisons.
Participants are invited to analyze and provide the BIPM with any results for measurement methods they apply for TET.HCl material characterization. The BIPM will provide a summary of these against the results obtained by STAGE 1A participants. The result review process will provide feedback where method optimization could be applied.
Reporting and use of results and submitted method descriptions
A reporting protocol will be developed by the BIPM for results and method descriptions. In submitting results and method descriptions, participants agree for the BIPM to use these in the development of an open access PEG. All contributing authors will be listed. This is a knowledge transfer study, and discussion and technical exchange between participants is encouraged before, during and in the analysis of the measurement results.
Timeline
| Date | Action |
| June 2021 | Registration deadline |
| July 2021 | Study Materials delivered to BIPM |
| September 2021 | Study Material and result/method reporting form sent to participants |
| September 2021 – March 2022 | Characterization Studies at NMIs |
| April 2022 | Result reporting from participants |
| May 2022 | Video conference to discuss results and submitted method reports |
| July 2022 | Draft PEG for Tetracycline.HCl distributed for comment |
| September 2022 | PEG for Tetracycline.HCl published |

