CCQM-K11 and CCQM-K11.2
MEASURAND : Mass fraction of glucose in human serum
Degrees of equivalence Di and expanded uncertainty Ui (95% level of confidence) expressed in mg/g.
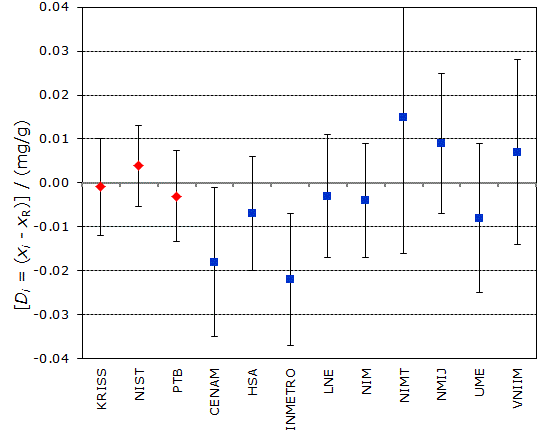
Red diamonds: CCQM-K11
Blue squares: CCQM-K11.2
CCQM-K11 and CCQM-K11.2
MEASURAND : Mass fraction of glucose in human serum
Degrees of equivalence Di and expanded uncertainty Ui (95% level of confidence) expressed in mg/g.
Information is available in Summary Results
| Metrology area, Sub-field | Chemistry and Biology, Organics |
| Description | Glucose in human serum |
| Time of measurements | 2001 |
| Status | Approved for equivalence |
| Final Reports of the comparisons | |
| Measurand | Mass fraction |
| Transfer device | Samples from two frozen human serum pools |
| Comparison type | Key Comparison |
| Consultative Committee | CCQM (Consultative Committee for Amount of Substance) |
| Conducted by | CCQM (Consultative Committee for Amount of Substance) |
| Comments | Two materials were used for the key comparison CCQM-K11. At its 8th meeting in 2002, the CCQM made the decision that only results for Material I would be included in the BIPM key comparison database (see Final Report).
Results published on 30 May 2003 Glucose in human serum The CCQM-K11.2 results are linked to the CCQM-K11 results.
|
| Pilot institute |
NIST
National Institute of Standards and Technology United States |
| Contact person | M.J. Welch +1 301 975 3100 |
This page proposes print-out on A4 paper (portrait) of the comparison details (best printed out using a black and white printer).
Please, select items to be printed out, then click on "OK" :
CCQM-K11 and CCQM-K11.2
MEASURAND : Mass fraction of glucose in human serum
CCQM-K11
|
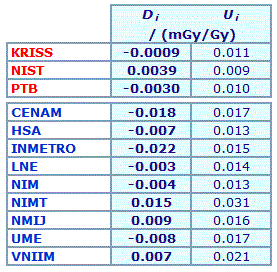
The key comparison reference value, xR, is calculated as the mean of the participant results; xR = 0.7762 mg/g. |
| The degree of equivalence of each laboratory with respect to the key comparison reference value is given by a pair of terms: Di = (xi - xR) and Ui, its expanded uncertainty corresponding to a 95% confidence interval, both expressed in mg/g. |
Linking CCQM-K11.2 to CCQM-K11
An independent key comparison reference value was assigned from the mean, standard deviation, and pooled standard uncertainty of the results reported by the three reference laboratories and the nine participants. It was agreed that although all results would be used to calculate the KCRV, degrees of equivalence would not be estimated for the three reference laboratories KRISS, NIST and PTB.
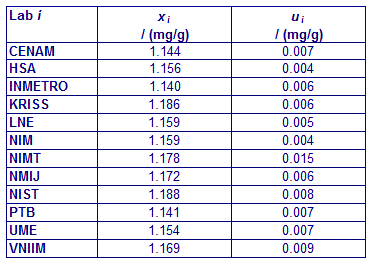
CCQM-K11 and CCQM-K11.2
MEASURAND : Mass fraction of glucose in human serum
xi : result of measurement carried out by laboratory i
ui : combined standard uncertainty of xi
CCQM-K11
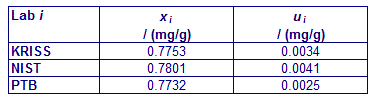
CCQM-K11.2