

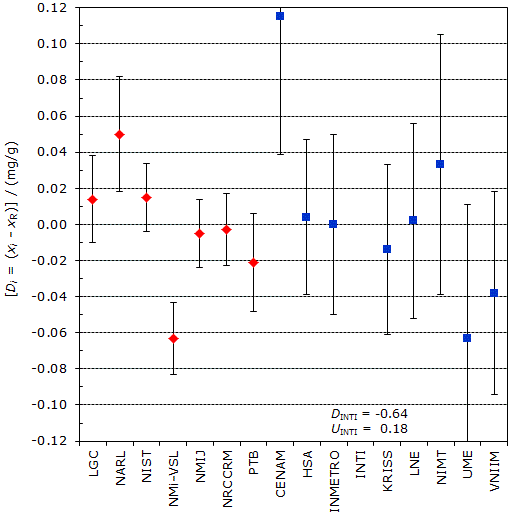
CCQM-K6 and CCQM-K6.2
MEASURAND : Mass fraction of cholesterol in human serum
2.2 mg/g to 2.3 mg/g (Matrial "A")
Degrees of equivalence Di and expanded uncertainty Ui (95% level of confidence) expressed in mg/g
 CCQM-K6
CCQM-K6
CCQM-K6.2
MEASURAND : Mass fraction of cholesterol in human serum
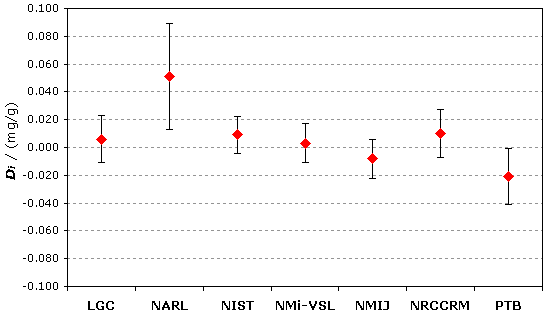
Material B : physiological range, ~1.7 mg/g
Degrees of equivalence Di and expanded uncertainty Ui (95% level of confidence) expressed in mg/g (xR = 1.726 mg/g, UR = 0.013 mg/g)
CCQM-K6 and CCQM-K6.2
MEASURAND : Mass fraction of cholesterol in human serum
2.2 mg/g to 2.3 mg/g (Matrial "A")
CCQM-K6
CCQM-K6.2
The full matrix of equivalence is available in Summary Results (summary of the results as a .PDF file).
MEASURAND : Mass fraction of cholesterol in human serum
Material B : physiological range, ~1.7mg/g
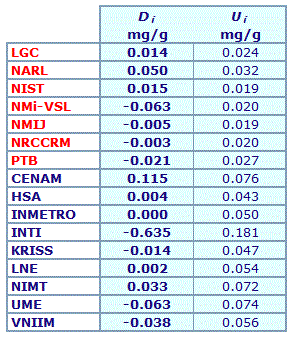
| Lab i | Di | Ui |
| / (mg/g) | / (mg/g) | |
| LGC | 0.006 | 0.017 |
| NARL | 0.051 | 0.038 |
| NIST | 0.009 | 0.013 |
| NMi-VSL | 0.003 | 0.014 |
| NMIJ | -0.008 | 0.014 |
| NRCCRM | 0.010 | 0.017 |
| PTB | -0.021 | 0.020 |
The full matrix of equivalence is available in Summary Results (summary of the results as a .PDF file).
| Metrology area, Sub-field | Chemistry and Biology, Organics |
| Description | Cholesterol in human serum |
| Time of measurements | 2012 |
| Status | Approved for equivalence |
| Final Reports of the comparisons | |
| Measurand | Mass fraction: ~2.2 mg/g and ~1.7 mg/g |
| Transfer device | Samples from two frozen human serum pools |
| Comparison type | Key Comparison |
| Consultative Committee | CCQM (Consultative Committee for Amount of Substance) |
| Conducted by | CCQM (Consultative Committee for Amount of Substance) |
| Comments | This is a bilateral key comparison subsequent to CCQM-K6. Results published on 07 June 2018 Cholesterol in human serum The CCQM-K6.2 results are linked to the CCQM-K6 results.
|
| Pilot institute |
NIST
National Institute of Standards and Technology United States |
| Contact person | M.J. Welch +1 301 975 3100 |
| Pilot laboratory | |
|---|---|
| NIST |
National Institute of Standards and Technology, United States, SIM |
| CENAM |
Centro Nacional de Metrologia, Mexico, SIM |
| HSA |
Health Science Authority, Singapore, APMP |
| INMETRO |
Instituto Nacional de Metrologia, Qualidade e Tecnologia, Brazil, SIM |
| INTI |
Instituto Nacional de Tecnologia Industrial, Argentina, SIM |
| KRISS |
Korea Research Institute of Standards and Science, Korea, Republic of, APMP |
| LNE |
Laboratoire national de métrologie et d'essais, France, EURAMET |
| NIMT |
National Institute of Metrology (Thailand), Thailand, APMP |
| UME |
TÜBITAK Ulusal Metroloji Enstitüsü, Türkiye, EURAMET |
| VNIIM |
D.I. Mendeleyev Institute for Metrology, Rosstandart, Russian Federation, COOMET |
This page proposes print-out on A4 paper (portrait) of the comparison details (best printed out using a black and white printer).
Please, select items to be printed out, then click on "OK" :
CCQM-K6 and CCQM-K6.2
MEASURAND : Mass fraction of cholesterol in human serum
2.2 mg/g to 2.3 mg/g (Matrial "A")
|
The key comparison reference value, xR, is calculated as the mean of the participant results excluding NARL and NMi-VSL; xR = 2.200 mg/g. |
| The degree of equivalence of each laboratory with respect to the key comparison reference value is given by a pair of terms: Di = (xi - xR) and Ui, its expanded uncertainty corresponding to a 95% confidence interval, both expressed in mg/g. |
Linking of CCQM-K6.2 to CCQM-K6
The linking was made via NIST, participating in both comparisons. Regarding the long time laps between the CCQM-K6 and CCQM-K6.2, a consensus value was applied as the key comparison reference value.
MEASURAND : Mass fraction of cholesterol in human serum
Material B : physiological range, ~1.7mg/g
|
The key comparison reference value, xR, is calculated as the mean of the participant results excluding NARL; xR = 1.726 mg/g. |
| The degree of equivalence of each laboratory with respect to the key comparison reference value is given by a pair of terms: Di = (xi - xR) and Ui, its expanded uncertainty corresponding to a 95% confidence interval, both expressed in mg/g. |
| The degree of equivalence between two laboratories is given by a pair of terms: Dij = Di - Dj = (xi - xR) - (xj - xR) = (xi - xj) and Uij, its expanded uncertainty corresponding to a 95% confidence interval, both expressed in mg/g. |
CCQM-K6 and CCQM-K6.2
MEASURAND : Mass fraction of cholesterol in human serum
2.2 mg/g to 2.3 mg/g (Matrial "A")
xi : result of measurement carried out by laboratory i
ui : combined standard uncertainty of xi
CCQM-K6
CCQM-K6.2
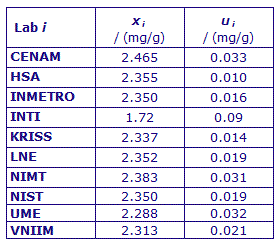
MEASURAND : Mass fraction of cholesterol in human serum
Material B : physiological range, ~1.7 mg/g
xi : result of measurement carried out by laboratory i
ui : combined standard uncertainty of xi
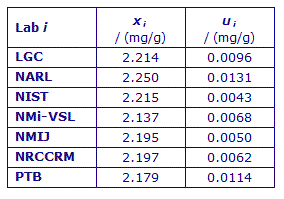
| Lab i | xi / (mg/g) |
ui / (mg/g) |
| LGC | 1.732 | 0.0066 |
| NARL | 1.777 | 0.0170 |
| NIST | 1.735 | 0.0033 |
| NMi-VSL | 1.729 | 0.0045 |
| NMIJ | 1.718 | 0.0039 |
| NRCCRM | 1.736 | 0.0062 |
| PTB | 1.705 | 0.0086 |